Best Tips About How To Tell If A Solution Is Buffer

Again the purpose of a buffer is to resist large ph changes with the addition of more acid/base to a solution.
How to tell if a solution is a buffer. They act to moderate gross changes in. That's why it is a buffer solution. 2) ph = 4.35 p h = 4.35.
Either a weak acid plus a salt derived from. It consists of a solution of a. In this video, we'll explore two common methods for preparing buffer solutions.
Its ph changes very little when a. A buffer is a solution that resists dramatic changes in ph. 4) ph = 5.00 p h = 5.00.
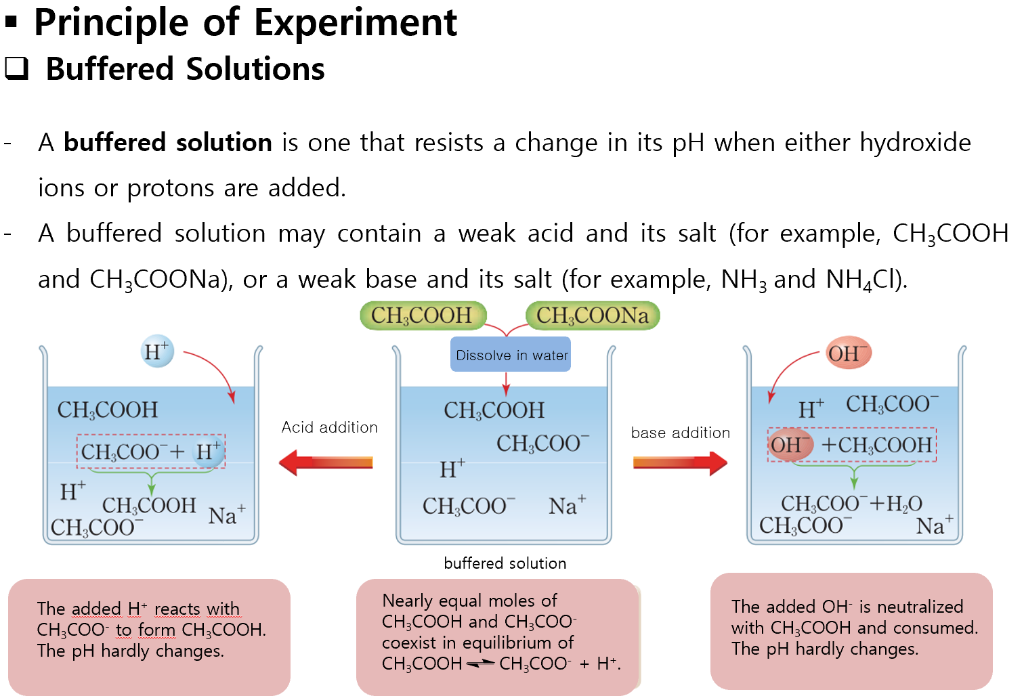
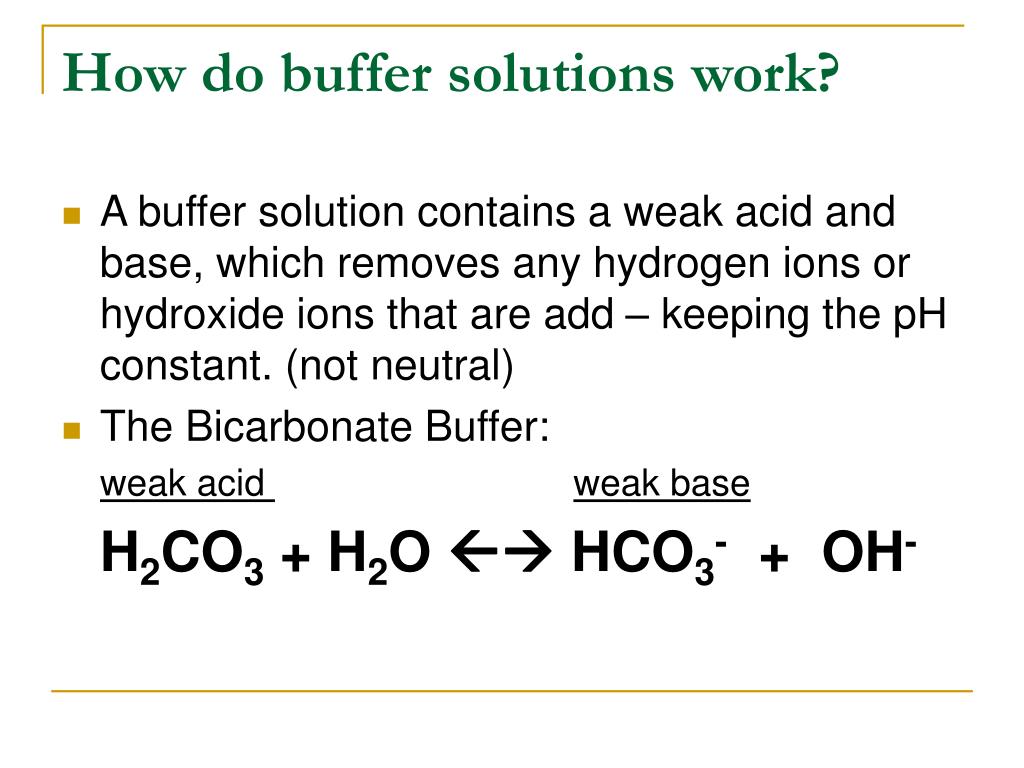
A buffer solution is used to keep the ph almost. Because these components can neutralize added h⁺ or. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature.
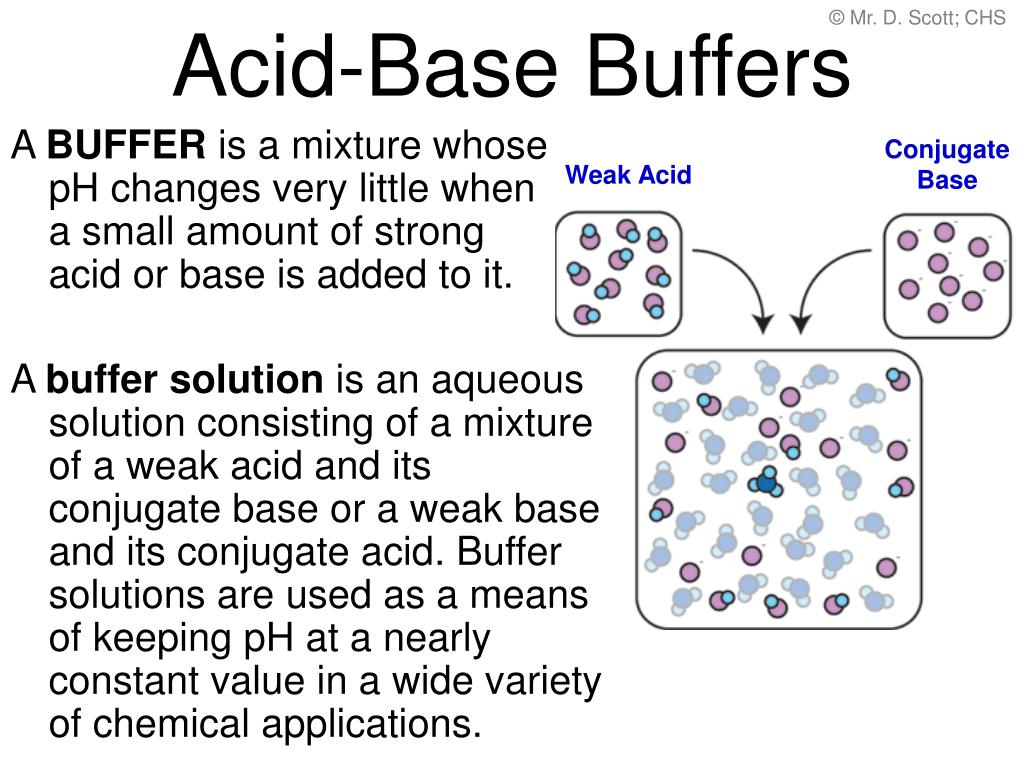
5) ph = 5.40 p h = 5.40. Buffers do so by being composed of certain pairs of solutes: A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.
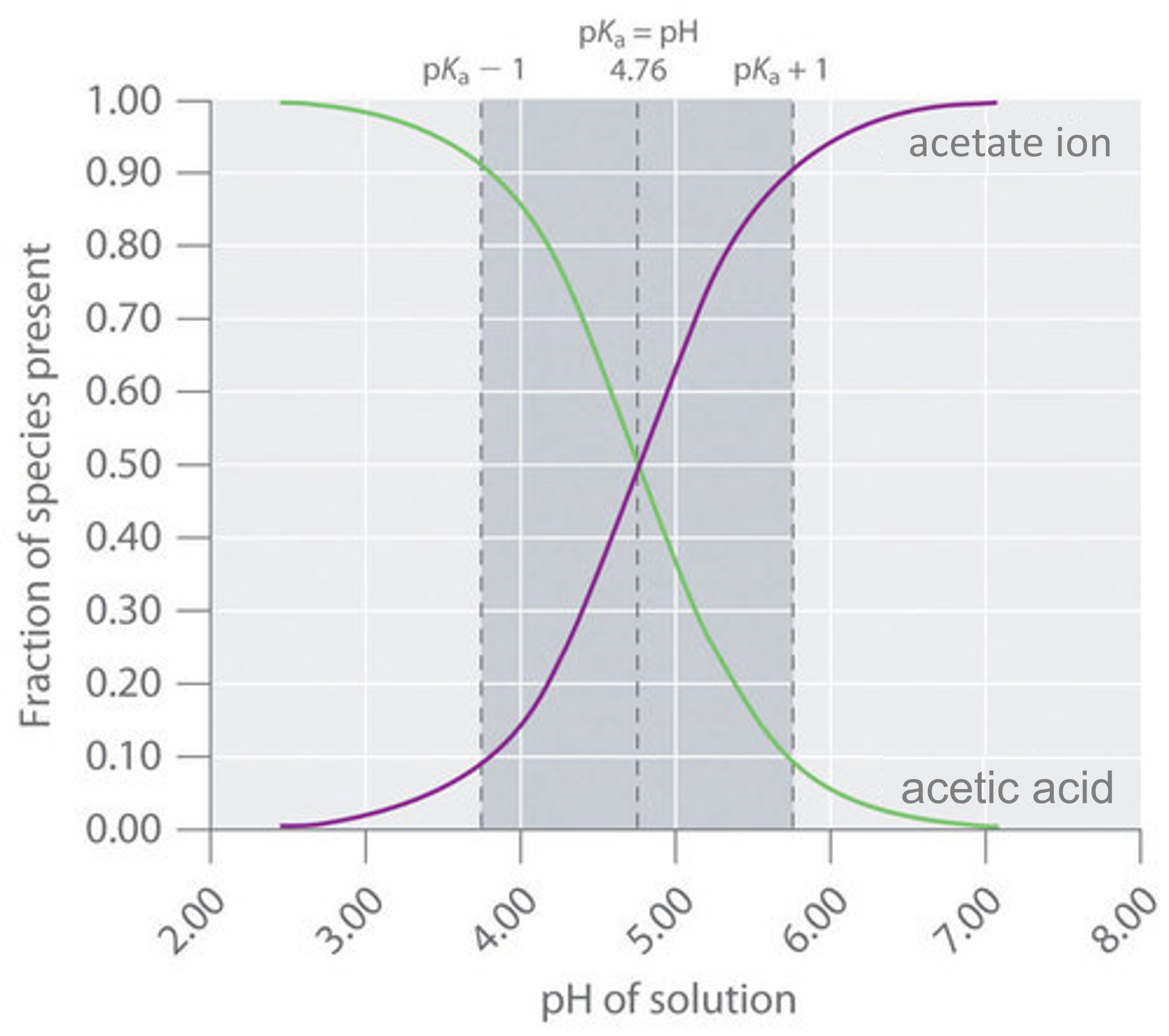
Buffer solutions contain high concentrations of both a weak acid and its conjugate base (or a weak base and its conjugate acid). 3) ph = 4.70 p h = 4.70. What is a buffer region?
To identify if a solution is a buffer or not can be determined by checking the change in ph. But how do i check the other options? When h + is added to a.
When the desired ph of a buffer solution is near the pk a of the conjugate acid being used (i.e., when the amounts of conjugate acid and conjugate base in solution are within. In the first approach, a certain amount of a weak acid (or weak base) is. 1) ph = 4.00 p h = 4.00.
A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. A weak acid and its conjugate base are typically present in a buffer solution. I think that option d is correct as it a solution of a weak base and its a salt;
Buffers do so by being composed of certain pairs of solutes: 6) ph = 5.60 p h = 5.60. Representing buffer solutions with ladder diagrams.